What is generic: affordable medicines or pharmaceutical waste?
Educational Program Health / / December 19, 2019
What are generic drugs?
Generic (Eng. generic, reproduced drug) - a drug-copy, which coincides with the original amount of the active ingredient and the influence on the body.
When inventing a new medication, his long research and testing, and then draw up a patent. When the patent expires, other companies can also produce such drugs - generics. But in the Russian patent holders rights are often violatedAbout intellectual property issues on the drug marketAnd generics are registered and sold even before the end patent for the original medicine.
In generics complicated names?
Not necessary. Every medication a few names: chemical, International Non-proprietary Name (INN) and sales.
Chemical name - it is unpronounceable phrase that does not speak about what you. INN - a unique name of the active substance, which is approved by WHO and must be indicated on the packaging of the drug.
In addition, the manufacturer of the drug may assign its product brand name to be written on the packaging in large letters.
Example:
- Chemical name: 2- (2- (2,6-Dihlorfenilamino) phenyl) acetic acid (as sodium salt).
- INN: diclofenac.
- Trade names: "Voltaren", "Vurdon", "Diklak", "Dikloberl", "Olfen," "Ortofen" and many others.
Why people choose generics?
Because they are much cheaper. Before patenting a new drug manufacturers spend huge money on its development and testing, and this affects the final cost. The procedure of registration of generic drugs is much easier and faster. This explains their low cost.
And there is no research?
According to lawArt. 18 of the Federal Law of 12.04.2010 N 61-FZ (ed. from 28.12.2017) "On Circulation of Medicines" for registration of generics instead of the report on its own preclinical studies can provide an overview of scientific papers on the results of preclinical studies reproducible preparation, and instead report on their own clinical trials - a report on the results of bioequivalence studies reproduced medications.
Bioequivalence shows the extent and rate of absorption, the time to reach maximum blood concentration distribution in tissues and body fluids, and rate of excretion.
So studies that prove the effectiveness and safety of a new generic, still held, but they are not as long-term and expensive, as in the case of the original drug.
And a lot of generics on the market?
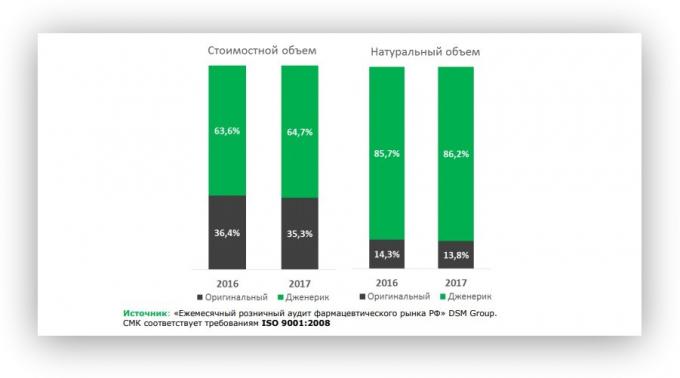
according to the reportRussian Pharmaceutical Market, December 2017 Analytical DSM Group, as in 2017 in the Russian market was 86.2% of generic drugs. A is 0.5% more than in 2016-m.
20.1% of all the sold generics - drugs affecting the digestive tract and metabolism, 14.2% - drugs to treat diseases of the nervous system, 14.0% - for the treatment of diseases of the cardiovascular system.
What about their efficiency?
StudyGeneric statins: Is everything so simple with evidence of clinical equivalence? 2012 showed that of the four generic simvastatin (medicines for lowering cholesterol), only two fully comply with the original safety and efficacy.
And in 2013 it became clearSafety and efficacy of generic drugs with respect to brand formulationThat due to the reduced effectiveness of generic drugs may increase the duration of the treatment or even not yield results. On the other hand, if you increase the dose of medication to speed up treatment, can cause negative effects.
It turns real lottery: some generic as effective and safe as the original, while others may extend treatment and cause side effects.
Why are generic drugs may be less effective?
On the effectiveness of the drug is influenced by many factors, including the degree of purification of the active substance and additional components, which may include generics. If the company buys the cheap active ingredient, the generic may not be sufficiently effective. A further components can cause allergy or side effects.
How to distinguish quality generic?
First of all, you can be guided by the price. If the drug is very cheap, even compared with other generics, its manufacturer is clearly on something saved. For example, the quality of the active substance or to control during manufacture.
A good indicator of the quality of products: presence GMP certificate (Good Manufacturing Practice) in pharmaceutical production. If the company has such a certificate, it means that its products are manufactured in the required conditions (cleanliness, temperature, humidity), in medicine do not get the extra material is packed properly and saves all their properties.
Should I use the generics?
Given the share of generics in the Russian market, we can say that all we have ever been treated by such drugs and nothing wrong with that. Generics make treatment available to a person with any income and provide a therapeutic effect and relative safety.
Writing out a prescription, the doctor specifies the name of the active ingredient, so you can independently select a specific generic and original drug (list of original products and generics can to find here). Often, the doctor advises a certified generic, then it better and use. If the drug makes the side effects, you should consult with your doctor: he may prescribe a more expensive generic or original drug.
see also
Homeopathy is officially recognized the danger →
Nootropics: what it is, whether they drink their work and costs →
Why you do not need antiviral drugs →
When to drink antibiotics, and when not →