What do we know about the first Russian drug for coronavirus
Health / / December 29, 2020

N + 1 - popular science edition about what is happening in science, engineering and technology right now.
The first Russian drug for coronavirus disease "Avifavir" received approval from the Ministry of Health. The developer of the drug promises that it will send the first batches of its drug to hospitals on June 11. We figure out where these yellow pills came from, on what principle the drug works, what clinical he has already managed to pass the tests and is it possible to say with certainty that we have a cure for COVID-19.
Where did it come from
Avifavir is a trade name for a Russian drug developed by the Russian Direct Investment Fund (RDIF) and the ChemRar group of companies. However, the active ingredient of the drug was not invented in Russia.
Its international non-proprietary name is favipiravir. Developed by employees of the Japanese company Toyama ChemicalToyama Chemical Co Ltd - a subsidiary of FUJIFILM Pharmaceuticals Corporation.
By its chemical structure, favipiravir is a derivative
Favipiravir 6 ‑ fluoro ‑ 3 ‑ oxo ‑ 3,4 ‑ dihydropyrazine ‑ 2 ‑ carboxylic acid, or pyrazinecarboxamide. During a screening of a chemical library, Toyama employees discovered that this substance may have activity against the influenza virus: cells infected with a virus, favipiravir is converted into an activated form that inhibits the activity of an important viral enzyme, RNA-dependent RNA-polymeraseCoronavirus disease 2019 (COVID-19): Management in hospitalized adults.If RNA polymerase is turned off, influenza viruses lose their ability to “print” their genetic material, RNA, in infected cells. As a result, the production of a virus that has already entered the cells stops. This is the uniqueness of the drug - usually antiviral drugs can only prevent viruses from entering cells.
RNA-dependent RNA-polymerase is not only found in viruses flu, but also in all RNA viruses. Moreover, the catalytic domain of RNA polymerase - this is the name of the part of the molecule, thanks to which the enzyme can, in principle, work - has the same structure in all RNA viruses. And since favipiravir binds precisely to the catalytic domain of RNA polymerase, the Japanese had reason to consider this substance an antiviral agent of a wideFavipiravir (T ‑ 705), a broad spectrum inhibitor of viral RNA polymerase spectrum of action.
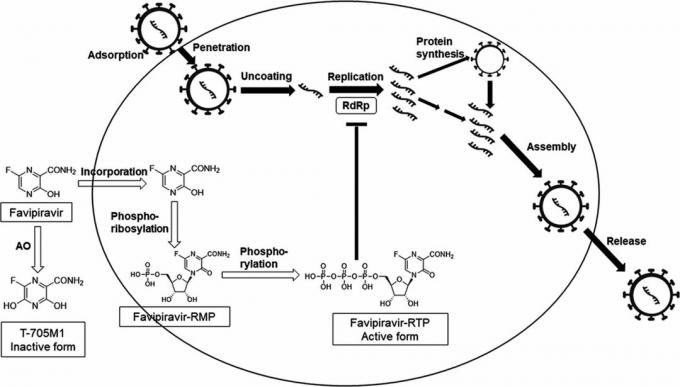
Toyama employees registered favipiravir under the brand name Avigan and began to investigate the activity of a promising drug on RNA virusesFavipiravir (T ‑ 705), a Novel Viral RNA Polymerase Inhibitor: from influenza viruses type A and B to Ebola virus. The results were mixed. For example, in the case of the Ebola virus, it turned out that the drug worked on monkeysAntiviral efficacy of favipiravir against Ebola virus: A translational study in cynomolgus macaques, but when applied on humans, the resultSurvival times improved in favipiravir ‑ treated Ebola patients was not very impressive. On the one hand, the mortality rate in 73 Guinean patients treated with favipiravir was lower than in patients who tried to heal in other ways. On the other hand, the difference was not that great: 42.5 percent versus 57.8 percent - that is, impossible ensure that this is not just a random counting artifact associated with the fact that the sample of patients was very small. Nevertheless, the Government of Guinea approvedJapan sending Fujifilm's flu drug favipiravir to over 40 countries for Covid-19 trials this drug is used as a standard treatment for the Ebola virus.
In the homeland of the drug, in Japan, Avigan succeeded register only in 2014 - and only against new strains of the influenza virus. Avigan has not been used against seasonal flu.

Moreover, the drug was approved not just against the "new" flu, but exclusively for situations when existing antiviral drugs were ineffective - that is, as a last resort. For six years from the moment of approval, such a situation has not arisen even once, so that in the conditions of a real flu epidemic, the drug was never used.
A review of 29 clinical trials (4,299 participants), six of which were phase 2 and 3 trials (already being evaluated) drug efficacy) showed that favipiravir “exhibits a favorable safety profile”: the proportion of serious side effects made upA Review of the Safety of Favipiravir - A Potential Treatment in the COVID-19 Pandemic? 0.4 percent. Still security problems drug still remain.
Japanese researchers studying the prospects of using the drug for severe influenza emphasizedFavipiravir, an anti ‑ influenza drug against life ‑ threatening RNA virus infectionsthat Avigan is contraindicated in pregnant women: the drug had teratogenic and embryotoxic effects on animals. Other possible problemsFavipiravir (United States: Not commercially available; refer to Prescribing and Access Restrictions): Drug information: decreased appetite, nausea, vomiting, increased concentration of uric acid in the blood (hyperuricemia) and liver damage.
Reading now🔥
- The consequences of the coronavirus can be lifelong. Here's what is known about it
Favipiravir and COVID-19
In March 2020, Zhang Xinmin, director of the National Center for Biotechnology Development, which is part of the Chinese Ministry of Health, saidFavipiravir shows good clinical efficacy in treating COVID-19: officialthat favipiravir “has shown good clinical efficacy against the novel coronavirus disease (COVID-19)”. According to the dataExperimental Treatment with Favipiravir for COVID ‑ 19: An Open ‑ Label Control Study at least one open-label, non-randomized study, 35 Chinese patients with coronavirus disease treated with favipiravir (the study did not say about which drug was discussed - the original Avigan or a Chinese medicine with the same active ingredient), recovered faster and suffered less complications than 45 patientswho were treated with other medicines (lopinavir and ritonavir).
Currently, the effectiveness of the drug against COVID-19 is being evaluatedCoronavirus disease 2019 (COVID-19): Management in hospitalized adults in clinical trials in Japan. April 9 FUJIFILM announcedFujifilm Announces the Start of a Phase II Clinical Trial of its Influenza Antiviral Drug “Avigan® Tablet” for COVID-19 Patients in the U.S. on the beginning of the second phase of clinical trials of Avigan, which will take place in the United States, 50 patients with coronavirus disease will take part in the trial. According to some foreign data, in April-May, favipiravir was tested in 16 more clinical trials, but none completed clinical trial showing that favipiravir or Avigan is effective against coronavirus disease, no.
Russian drug
Any medicine consists of an active substance and a filler (finished dosage form). Russian antiviral drug contains the same active ingredientFirst Russian drug against coronavirus received approval from the Ministry of Healthas the Japanese drug - that is, 200 milligrams of favipiravir per tablet. As in a conversation with "N + 1" pointed out by the representative of the RDIF Arseny Palagin, the filler of the Russian drug is its own. The instructions say that the excipients include microcrystalline cellulose, croscarmellose sodium, colloidal silicon dioxide, magnesium stearate and povidone K-30. The patent protection period for the original Japanese Avigan expired in 2019, so the drug can be considered legally produced. generic.
Russian clinical trials of Avifavir are also not finished yet. Completed only the first and second stages of a multicenter randomized study, confirms the interlocutor "N + 1" from the RDIF. The first stage involved 60 people - 20 of them were included in the control group, which was treated with standard methods. Data on the age composition and severity of the subjects' condition were not disclosed.
Here's what is reportedRDIF and ChemRar Group of Companies will deliver 60,000 courses of Avifavir, the first drug registered in the Russian Federation against COVID-19, to Russian hospitals in June the developers themselves about the results of these tests:
- new side effects in addition to those that the Japanese recorded many years ago, they did not reveal;
- after four days of treatment in 65 percent of people in the experimental group coronavirus test gave a negative result (in the control group, such cases were about 30 percent);
- after three days, 68 percent of people in the experimental group returned to normal temperature (in the control, this happened on the sixth day).
The Ministry of Health approved the start of the third phase of the Avifavir trials on May 1, 2020. At this stage, according to the dataState Register of Medicines on the website of the State Register of Medicines, a total of 390 people will have to take part. The same data show that favipiravir is being tested by two more Russian companies: Drugs Technology (part of the R-Pharm group) and Promomed. Both companies began testing at the end of May.
Although the trials are not completed and only partial data on the effectiveness are known, the Ministry of Health allowed the drug to be registered early - according to the accelerated procedure adopted under the decreeResolution of the Government of the Russian Federation of April 3, 2020 No. 441 Government of the Russian Federation dated April 3, 2020 No. 441. This decree says that "a reduction in the volume <...> of examinations" is permissible "in conditions of the threat of the emergence and liquidation emergency».
So the manufacturer already promisesRDIF and ChemRar Group of Companies will deliver 60,000 courses of Avifavir, the first drug registered in the Russian Federation against COVID-19, to Russian hospitals in June by the end of next week, bring the first batches of pills to hospitals.
In the instructionsInstructions for the medical use of the drug "Avifavir" to the drug it is written that it was "prepared on the basis of a limited amount of clinical data on the use of the drug and will be supplemented as new data becomes available." However, contraindications have already been identified. As with the Japanese Avigan, this is pregnancy planning, pregnancy and the period of breastfeeding - the generic is also potentially teratogenic. Patients with gout and hyperuricemia should use the medication with caution. In addition, the list of contraindications to the Russian generic drug was supplemented by hypersensitivity to the active substance, age up to 18 years, severe hepatic and renal failure.
The drug will not be delivered to pharmacies: according to the instructions, the drug can be used only in hospitals.
What is the result
Avifavir is a generic drug of Favipiravir, which has a distinct mechanism of action and is undergoing clinical trials in Russia and abroad.
The results of interim clinical trials show that the drug is promising: on those small samples that they managed to check, the therapeutic effect, judging by the statements of the developers, to determine succeeded. But until clinical trials are completed and the results are published in peer-reviewed international magazines, we cannot be completely sure that Avifavir really helps against coronavirus disease. The press service of ChemRar, the drug's developer, did not answer the questions of N + 1 at the time of publication of this text.
Now we do not have drugs that would purposefully and effectively act against the SARS ‑ CoV ‑ 2 virus. All contenders for this title now are substances known before the emergence of the new coronavirus, which in clinical tests (which have just begun) are consistently showing some positive effect with a number of restrictions. There are two of them right now.
The first is Remdesivir, which hit the front pages in May, a drug that even before the COVID-19 epidemic was intended to treat another coronavirus infection, Middle East Respiratory Syndrome (MERS). American regulator approved the clinical use of Remdesivir without waiting for formal completion drug trials, - the presence of a stable effect in preliminary research data convinced medical officials. This situation was comparedAll eyes on gilead with early registration AZTZidovudine, the first cure for HIV.
At the same time, "Remdesivir" never claimed the status of "silver bullet": tests showRemdesivir for the Treatment of Covid-19 - Preliminary Reportthat it cannot help people with severe symptoms - who need mechanical ventilation of the lungs, and for those with milder symptoms, it reduces the time of illness by four days. Many other effects - for example, a decrease in the mortality of the disease compared to other drugs - were not shown with statistical significance in these trials. The sample of Remdesivir trials, which were reported in late May by the New England Journal of Medicine, was 1,059 people.
Elena Verbitskaya
Head of the Department of Biomedical Statistics of St. Petersburg State Medical University named after academician I. P. Pavlova.
60 subjects - is that a lot or a little?
The number of subjects required to test the effectiveness of a drug is calculated using special formulas that take into account many variables: for example, features of the indicators that are supposed to be taken into account, their spread, the level of deviation from the indicators of the control group, which will be considered clinically significant.
Indicators are assigned that will be taken into account during the tests. The main one is usually mortality. In the case of respiratory infections, integral indicators are used, which take into account, for example, the number of days with fever, time in intensive care or intensive care unit, on mechanical ventilation, and cough. All of them are converted into points according to a certain formula, and then the points of the experimental group are compared with the points of the control group.
For some research, 20 subjects will be enough. For some, 2000 is not enough.
Pilot trials in a small group may be conducted prior to clinical trials. It is not uncommon for a situation when an effect found in a group of several dozen people subsequently “erodes” in large groups.
Favipiravir, like Remdesivir, was not originally developed as a remedy specifically against the new coronavirus. The drug many years ago - so much so that the patent for it has already expired - was adapted for the treatment of influenza (strictly new viruses, not seasonal diseases) and tested against Ebola and Zika viruses.
Yes, Russian researchers seem to have caught the effect of its use in treatment COVID-19 - but so far on a small sample of 60 people, there is no detailed information on the selection methods and composition of which.
So we seem to have pills. And to make sure that this is really a medicine, you will have to wait a little longer.
Coronavirus. Number of infected:
6 661 888
in the world441 108
in RussiaRead also🦠
- How long will immunity to the new coronavirus last?
- 6 tricks that don't really ward off germs
- What new waves of a pandemic might look like



