The new principle of cancer treatment, has earned the Nobel Prize in 2018
Forming / / December 19, 2019
After the Nobel Committee for two consecutive years were presented in Physiology or Medicine Prize for purely fundamental research, this time he finally lived up to expectations and awarded the prize for cancer immunotherapy. The prize will share American immunologist James Ellison and his Japanese counterpart Tasuku Honjo. Selecting Committee surprised no one - at least, the name of James Ellison last few years consistently falls into the "short list" of nominees according to journalists.
In 2016 Clarivate Analytics Agency, which is a forecast based on quoting of scholars, included it to your list of potential winners. Ellison Honjo development and led to the emergence of a new principle of treatment of malignant tumors, based on the ability of our own immune system to recognize and destroy cancer cells.
immunity brakes
Job immune cells, in this case - T-lymphocytes on the verge between "nedosledit" and "overreach". To maintain balance and prevent autoimmune diseases when cells begin to mistakenly attack normal cells, lymphocytes have a "gas" and "brake". This protein receptors on the cell surface, the binding of which certain molecules or ligands activates or inhibits the activity of immune system to destroy the potential "enemies".
Collectively, these regulators are called immune control pointsOr "Checkpoint". In each group of "checkpoints" (stimulant and immunity inhibitors) includes more than a dozen molecules, but here we will look at two of them - CTLA-4 and PD-1. Both of these molecules are the "brakes" of the immune system.
Cytotoxic T cells receive information about what they need to pay attention, from antigen-presenting cells that "show" lymphocytes fragments of foreign proteins. To activate the lymphocytes to tumor cells, antigen-presenting cell should contact not only with the T-cell receptor, but with co-stimulatory receptor CD-28. In CD-28 is the "evil twin brother» CTLA-4, which interacts with the same marker as the CD-28, but, on the contrary, the overwhelming immune response - namely, the T-cell proliferation and production of signaling molecules that are responsible for the implementation of the immune response. If the "switch off» CTLA-4, in the CD-28 will not be competitive and there will be activation of the immune system.
The idea that CTLA-4 acts "brake" of immunity, belongs, including Ellison. In 1994, the members of his laboratory at the University of California at Berkeley doing this receptor in the search for drugs against autoimmune diseases. However, Ellison had another idea: instead of strengthening CTLA-4, he proposed to block it using specific antibodies, and thus activate anti-tumor immunity in mice carcinoma. Suitable load - administering antibodies inhibited the growth of tumors in mice. In addition, cured mice developed immunity against this type of cancer - the reintroduction of malignant cells does not lead to tumor formation. Article published with the results of the experimentEnhancement of Antitumor Immunity by CTLA-4 Blockade in Science in 1996.
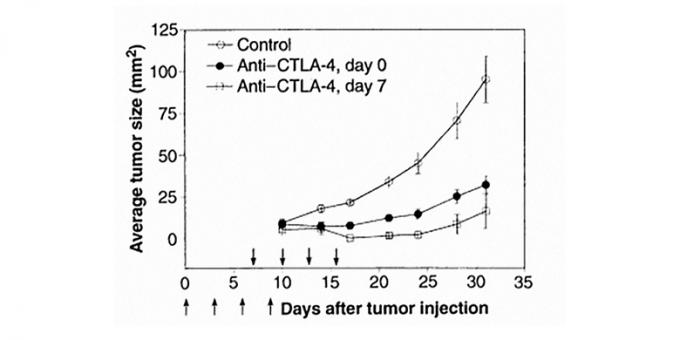
antibody treatment against CTLA-4 inhibits the growth of tumors in mice. Changes in tumor size in the control animals injected with irrelevant to the receptor antibody is indicated by white circles. Experimental groups were administered a therapeutic antibody simultaneously with tumor cells (black circles) or on the seventh day after injection of tumor cells (white squares).
These and similar experiments on CTLA-4 blockade caused interest in the industry, and a few years later approach has been tested in humans. In 2011, by the US Food and Drug Administration approved the first medical drug therapy aimed at malignant melanoma by inhibiting immune "checkpoint" - ipilimumabYervoy Approval History (A monoclonal antibody that binds to the receptor and giving it to operate). As long as this is the only approved drug, to work with CTLA-4.
For this work, Ellison was awarded numerous awards - among them, and the Lasker Award, the most prestigious, after the Nobel in medicine, and the National Foundation for Cancer Research Award, and awards from various pharmaceutical companies. We can say that Allison from 2011 annually receive some award, so this series is over Nobel quite natural.

The second "arm" of immunity - PD-1 - came to the clinic later, although there was opened several years earlier. This merit belongs to the employee of the Kyoto University in Japan Tasuku Honjo. He and his colleagues identifiedInduced expression of PD-1, a novel member of the immunoglobulin gene superfamily, upon programmed cell death PD-1 gene, found that the corresponding protein belongs to the immunoglobulin superfamily, and have shown that it is somehow linked to cell death in the tumor cell model.
The fact that PD-1 acts as a negative regulator of immunity, was setEngagement of the Pd-1 Immunoinhibitory Receptor by a Novel B7 Family Member Leads to Negative Regulation of Lymphocyte Activation in 2000 alone. Furthermore, in this work it was found a partner (ligand) PD-1 - a protein that is well named, PD-L1 (ligand of PD-1). It has been found that cancer cells often produce a lot of protein PD-L1 on their surface. The idea that blocking both PD-1 and PD-the L1 may lead to the activation of antitumor immunity in mice was confirmed in the publicationPD-1 blockade inhibits hematogenous spread of poorly immunogenic tumor cells by enhanced recruitment of effector T cells 2005.
Already in 2006 it was launched the first clinical trial in humans, however, the first anti-PD-1 drug was approved nivolumabOpdivo Approval History for the treatment of cancer only in 2014. At the same time it has been approved and a second drug - pembrolizumabKeytruda Approval History by the company Merck. Among the types of cancer against which the effect of anti-PD-1 antibody, - progressing melanoma, non-small cell lung cancer, renal cell carcinoma, Hodgkin's lymphoma, and others.

The mechanism of action of inhibitors of PD-1 and PD-L1. T cell has on its surface a T-cell receptor (TCR) and PD-1 receptor, through which a lymphocyte interacts with both the antigen-presenting cell (APC), and with a cancerous cell, which has a protein PD-L1. Antibodies that block both PD-1 and PD-the L1, do not give a cancer cell "slow down" the immune response against it.
What has changed in the treatment of cancer
Until recently, cancer therapy is based on three main principles - surgery, irradiation of the tumor by radiation and anticancer chemotherapy, where the aim is a relatively non-specific, with few exceptions, the destruction of rapidly dividing tumor cells with toxic substances.
However, in the beginning of the XX century, scientists realized that the tumor cells - the same target immunity, bacteria and parasites, and have taken the first attempts to treat tumors of immune activation. Nevertheless, the idea of an anti-cancer immunity in some point refused. The discoveries made in laboratories Ellison and Honjo, radically changed this view, and therapy "Inhibitors checkpoints", particularly antibodies against PD-1 and PD-L1, is now at the forefront medicine.
"In clinical oncology is one of the biggest events in the history", - says Deputy Director General National Research Center of Pediatric Hematology, Oncology and Immunology named Dima Rogachev Mihail Maschan. "We are only now beginning to reap the benefits that brought the development of this type of therapy. Now it registered five (actually six. - Approx. Ed.), this group of drugs, and to develop there are dozens, if not hundreds of molecules that exploit this principle. "
What is the difference between these drugs from the more traditional methods of treatment? As shown by the first results of clinical trials on patients with an aggressive form of melanoma, the first approved therapy "checkpoint inhibitor" increasedLongest follow-up of largest number of melanoma patients treated with ipilimumab shows some survive up to ten years survival and a half times compared with standard chemotherapy. In addition, immunotherapy is assigned to patients in cases where the standard chemotherapy has not helped or illness returned. For example, among the types of cancer for which treatment is approved pembrolizumab - metastatic lung cancer and recurrent head and neck cancer. Many patients with such diagnoses chemotherapy is not helping.
"The fact that patients with widespread tumors, especially melanoma, is now a combination of these Checkpoint inhibitors of long-term survival, i.e. actually cure can be achieved in 30-40 percent cases. In certain other tumors, too, very much improved results ", - says Mikhail Maschan. "This is the beginning of the way, but we know many types of tumors - and lung cancer, and melanoma, and some others in which therapy demonstrated effectiveness, but even more - in which she only studied, researched its combination with conventional forms of therapy. The number of people who have survived tens of thousands of measured thanks to this therapy. "

not a panacea
Unfortunately, in spite of the promising results, the therapy "checkpoint inhibitors" does not help everyone. For example, a protein PD-L1 expressed by cancer cells, not all people, because immunotherapy directed against it will not work if there is no protein. There is evidence that this type of treatment is best to help patients who have for one reason or another increased the likelihood of mutations in DNA, including, under the influence of tobacco smoke or radiation. For example, the already mentioned pembrolizumab recommended as a therapy not operated or metastatic tumors in patients with impaired DNA repair. This is the first event in cancer therapy when the drug is recommendedTissue-independent cancer drug gets fast-track approval from US regulator for the treatment of tumors only based on the presence of certain mutations, regardless of tumor type.
"Cancer - hundreds if not thousands of different diseases, and each its own mechanisms and molecular pathways that while working, and no one will come up with a drug that is effective in all types of cancer in all patients. But these medicines help in different types of tumors, because they do not operate at the level of individual processes in the tumor cell, and at the level of the immune system, "- says Maschan.
In addition, the therapy is not without side effects. First of all, it is an autoimmune reaction, because the drugs cause activation of the immune system, "despise dogs with chains," as Michael Maschan. Treatment "checkpoint inhibitors" can lead to inflammation of various organs. In addition, the drugs work well in adults, but do not work on children. Finally, the treatment of as yet very, very expensive.
"This revolution is mainly touched adult oncology, these studies in pediatric oncology is not a revolution done, because children's tumors biologically constructed differently, the immune system sees them very badly, - he explains Maschan. - In Russia, officially approved for use three drugs in this group. But, unfortunately, the price of one course of therapy is quite high, it is measured in tens of thousands of dollars a year. However, the Russian biopharmaceutical company working to develop local versions of these drugs, and it is likely that in the near future they will appear on the market at lower prices. I think that this can be expected in a year -. Two "
read also
- 15 signs of cancer that can not be ignored women →
- 35 reasons why we are more fortunate than our parents →
- Medical procedures, the passage of which is not to forget →



